Scientific Papers
MycoKey aims to unlock new knowledge and to valorize existing knowledge and data by rapid dissemination to research partners and stakeholders in the chain.
In the MycoKey programme all scientific peer reviewed publications are available through open access. Research data will be deposited in public data repositories for (re)-analysis exploitation and dissemination free of charge.
In this page you can find publications describing the scientific results arising from project’s activities.
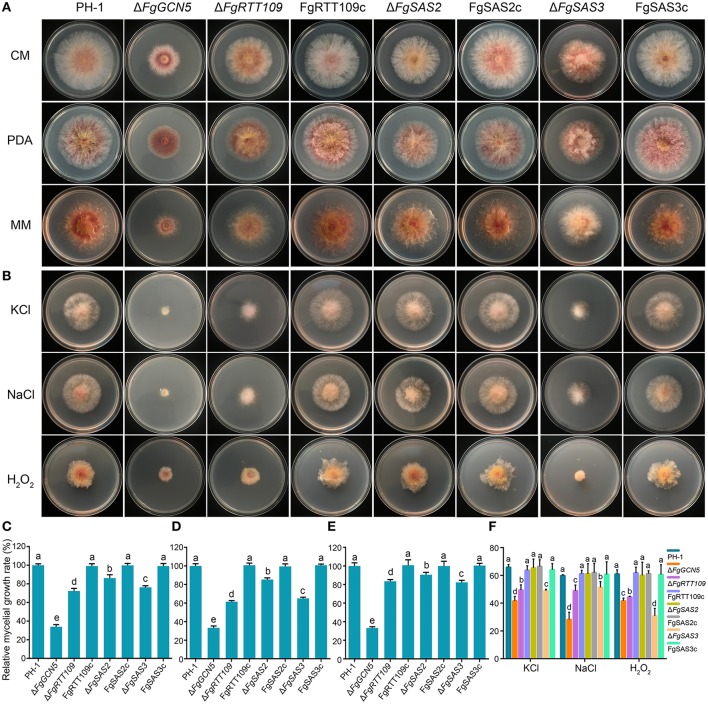
The Fusarium graminearum Histone Acetyltransferases Are Important for Morphogenesis, DON Biosynthesis, and Pathogenicity
- google+
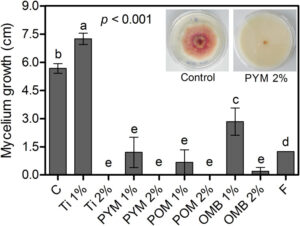
Post-translational modifications of chromatin structure by histone acetyltransferase (HATs) play a central role in the regulation of gene expression and various biological processes in eukaryotes. Although HAT genes have been studied in many fungi, few of them have been functionally characterized. In this study, we identified and characterized four putative HATs (FgGCN5, FgRTT109, FgSAS2, FgSAS3) in the plant pathogenic ascomycete Fusarium graminearum, the causal agent of Fusarium head blight of wheat and barley. We replaced the genes and all mutant strains showed reduced growth of F. graminearum. The ΔFgSAS3 and ΔFgGCN5 mutant increased sensitivity to oxidative and osmotic stresses. Additionally, ΔFgSAS3 showed reduced conidia sporulation and perithecium formation. Mutant ΔFgGCN5 was unable to generate any conidia and lost its ability to form perithecia. Our data showed also that FgSAS3 and FgGCN5 are pathogenicity factors required for infecting wheat heads as well as tomato fruits. Importantly, almost no Deoxynivalenol (DON) was produced either in ΔFgSAS3 or ΔFgGCN5 mutants, which was consistent with a significant downregulation of TRI genes expression. Furthermore, we discovered for the first time that FgSAS3 is indispensable for the acetylation of histone site H3K4, while FgGCN5 is essential for the acetylation of H3K9, H3K18, and H3K27. H3K14 can be completely acetylated when FgSAS3 and FgGCN5 were both present. The RNA-seq analyses of the two mutant strains provide insight into their functions in development and metabolism. Results from this study clarify the functional divergence of HATs in F. graminearum, and may provide novel targeted strategies to control secondary metabolite expression and infections of F. graminearum.
Authors: Xiangjiu Kong, Anne D. van Diepeningen, Theo A. J. van der Lee, Cees Waalwijk, Jingsheng Xu1, Jin Xu1, Hao Zhang1, Wanquan Chen1, Jie Feng1
Keywords: Fusarium graminearum; deoxynivalenol; histone acetyltransferase; pathogenicity; secondary metabolism
Published: 26/04/2018
Repository: https://www.ncbi.nlm.nih.gov/pubmed/29755419
This work has received funding from the National Key R&D Program of China (2016YFE0112900, 2016YFD0300705) and European Union’s Horizon 2020 research and innovation programme under grant agreement No 678781 (MycoKey) and Fundamental Research Funds for Central Non-profit Scientific Institution (Y2017XM01).CC-BY license http://creativecommons.org/